Every day, millions of people around the world take different medicines. With thousands of medications out there, how do healthcare professionals keep track of them all? How do they know which medicines are similar, or might cause the same side effects? This is where WHO Drug SDGs come in.
WHO Drug SDGs refer to the WHO Drug Standardized Drug Groupings. They are used in pharmacovigilance and clinical research for coding, analyzing, and interpreting drug information consistently.
Lets say you are maintaining a library of thousands of books. To help find a particular book, it will be easy if you divide the book-shelfs into categories such as Fiction, Non-Fiction, History, Cooking etc. Similarly, the data about medications/medicinal products is a huge collection. WHO Drug SDGs are the categorization system, grouping medicines that work in similar ways or treat the same type of illness. This makes it easier for doctors, researchers, and healthcare, PV teams to find and analyze drugs. Also, a book can be tagged with multiple categories — for instance, a book about traditional Chinese herbal medicine could appear under “Traditional Medicine,”…
Another analogy to understand it is to compare it with Netflix. Just like Netflix groups movies into categories like “Action,” “Romance,” and “Sci-Fi” — sometimes putting the same movie in multiple categories — WHODrug SDGs organize medicines based on how they work, what they’re made of, and what they treat.
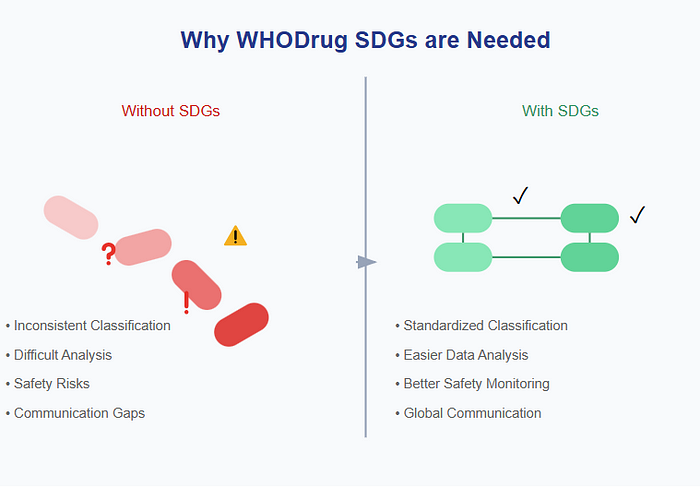
Imagine trying to track the information about thousands of medicines worldwide without a standardized way to group them. It would be like trying to organize a massive library without any system — chaos! WHODrug SDGs give researchers, doctors, and safety experts a common language to:
Track medicine safety
Spot patterns in side effects
Compare similar drugs
Make sense of safety data from different countries
The primary purpose of SDGs is to simplify and standardize data analysis in safety and efficacy studies, including pharmacovigilance, signal detection, and clinical trial evaluations. For anyone working in drug safety or clinical research, WHODrug SDGs are like having a universal translator. They help ensure that when someone in Japan reports a side effect, it can be properly compared with similar reports from Brazil or Nigeria. This global standardization is crucial for keeping medicines safe worldwide. The system helps answer questions like:
Are all drugs in this class causing similar side effects?
Is this new safety concern unique to one medicine or common across similar ones?
How do safety profiles compare across similar medicines?

WHO Drug Standardized Drug Groupings (SDGs) are organized using a hierarchical classification system known as Anatomical Therapeutic Chemical (ATC) Classification System. The ATC classification system has 5 hierarchical levels that progressively narrow down a drug’s classification from general to specific.
1st Level: Anatomical Group
Represents the organ or system the drug acts on.
Example: “N” for the Nervous System.
2nd Level: Therapeutic Main Group
Indicates the drug’s therapeutic purpose or general pharmacological action.
Example: “N05” for Psycholeptics (drugs that calm or sedate).
3rd Level: Pharmacological Subgroup
Further details the drug’s pharmacological effect
Example: “N05A” for Antipsychotics.
4th Level: Chemical Subgroup
Groups drugs with similar chemical characteristics or specific actions.
Example: “N05AH” for Atypical Antipsychotics.
5th Level: Chemical Substance
Specifies the individual active substance in the drug.
Example: “N05AH03” for Olanzapine.
N05AH03 is known as the ATC code for Olanzapine.
The breakdown for Olanzapine
N: Nervous system (Anatomical group)
N05: Psycholeptics (Therapeutic group)
N05A: Antipsychotics (Pharmacological subgroup)
N05AH: Atypical antipsychotics (Chemical subgroup)
N05AH03: Olanzapine (Chemical substance)
Remember that same drug may appear in multiple groupings. E.g Olanzapine can appear in:
Antipsychotic Medications (based on primary use)
Weight-Affecting Drugs (based on side effect profile)
Metabolic Impact Drugs (based on physiological effects)
This multi-dimensional grouping allows for more comprehensive safety analysis. Also, note that while SDGs use ATC codes as one way to group drugs, they also provide additional grouping possibilities beyond the ATC hierarchy.
The WHO Drug Standardized Drug Groupings (WHO Drug SDGs) are maintained and updated by the Uppsala Monitoring Centre (UMC), which is responsible for managing and developing the WHODrug Global database. The UMC is an independent, non-profit organization collaborating with the World Health Organization (WHO) to support global pharmacovigilance efforts. It works closely with regulatory authorities, pharmaceutical companies, and healthcare organizations to ensure the database meets international needs.
WHO Drug SDGs are updated regularly (typically quarterly). These updates reflect:
Addition of new drugs
Changes to existing drugs (e.g., new indications, updated classifications).
Retirements of obsolete drugs.
Feedback from users and pharmacovigilance experts
New drug approvals from regulatory bodies like the FDA, EMA etc
Changes in drug usage patterns or therapeutic areas
These updates are distributed via:
Updates are distributed to subscribers(pharmaceutical companies, regulatory bodies, CROs, etc.) via:
WHODrug Insight (a web-based tool).
Downloadable files in formats like B3 or C3 (used in database integrations).
WHODrug SDGs provide a universal language for drug classification, enabling healthcare professionals worldwide to communicate effectively about medications, their effects, and safety concerns. Through their comprehensive, standardized approach to drug classification, WHODrug SDGs continue to play a vital role in advancing global drug safety, supporting medical research, and ultimately improving patient care worldwide.